Details of the Drug
General Information of Drug (ID: DMB7WYM)
| Drug Name |
ATOSIBAN
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Atosiban; Tractocile; 90779-69-4; Atosiban acetate; Antocin; UNII-081D12SI0Z; Rwj 22164; Orf-22164; RWJ-22164; CHEMBL382301; 1-Deamino-2D-tyr-(OEt)-4-thr-8-orn-oxytocin; dTVT; 081D12SI0Z; 1-Deamino-2-D-Tyr-(O-ethyl)-4-Thr-8-ornoxytocin; 1-(3-Mercaptopropanoic acid)-2-(O-ethyl-D-tyrosine)-4-L-threonine-8-L-ornithineoxytocin; NCGC00165718-01; Atosibanum [INN-Latin]; ORF 22164; deTVT; Atosibanum; Antocin II; Atosiban [USAN:INN:BAN]; RWJ22164; 1-(3-Mercaptopropionic acid)-2-(3-(p-ethoxyphenyl)-D-alanine)-4-L-threonine-8-L-ornithineox
|
|||||
| ATC Code | ||||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
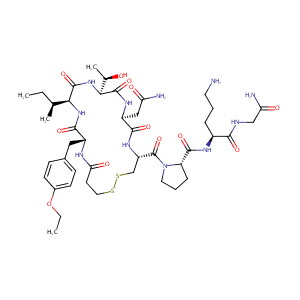 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 994.2 | ||||
| Logarithm of the Partition Coefficient (xlogp) | -1.9 | |||||
| Rotatable Bond Count (rotbonds) | 18 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 11 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
References
